Chlorogenic Acid: The Hidden Star Behind Your Coffee’s Flavor
Chlorogenic Acid & Coffee
If you’re a coffee lover like me, you’re likely familiar with acidity, bitterness, and body. But what gives coffee its distinct flavor profile? It’s chlorogenic acid, a potent polyphenol that not only shapes the taste of your brew but also contains many health benefits.
Before diving into chlorogenic acid, let’s explore polyphenols, nature’s antioxidant powerhouse. These plant-based compounds protect your body from free radicals, reduce inflammation, and support overall well-being. Ready to learn more? Let’s get started.
Types of Polyphenols:
Flavonoids: Found in fruits, vegetables, tea, and coffee. Examples: quercetin, catechins, anthocyanins.
Phenolic Acids: Present in coffee, tea, and berries. Includes chlorogenic acid and caffeic acid.
Polyphenolic Amides: Found in chili peppers and oats. Example: capsaicin.
Other Polyphenols: Includes resveratrol (red wine), curcumin (turmeric), and tannins (tea, coffee).
Health Benefits:
Antioxidant Protection: Neutralizes free radicals, preventing cell damage.
Anti-inflammatory: Reduces inflammation and lowers the risk of chronic diseases.
Heart Health: Improves blood circulation and reduces blood pressure.
Blood Sugar Regulation: Helps manage blood sugar levels.
Brain Health: May protect against neurodegenerative diseases.
Polyphenols in Coffee:
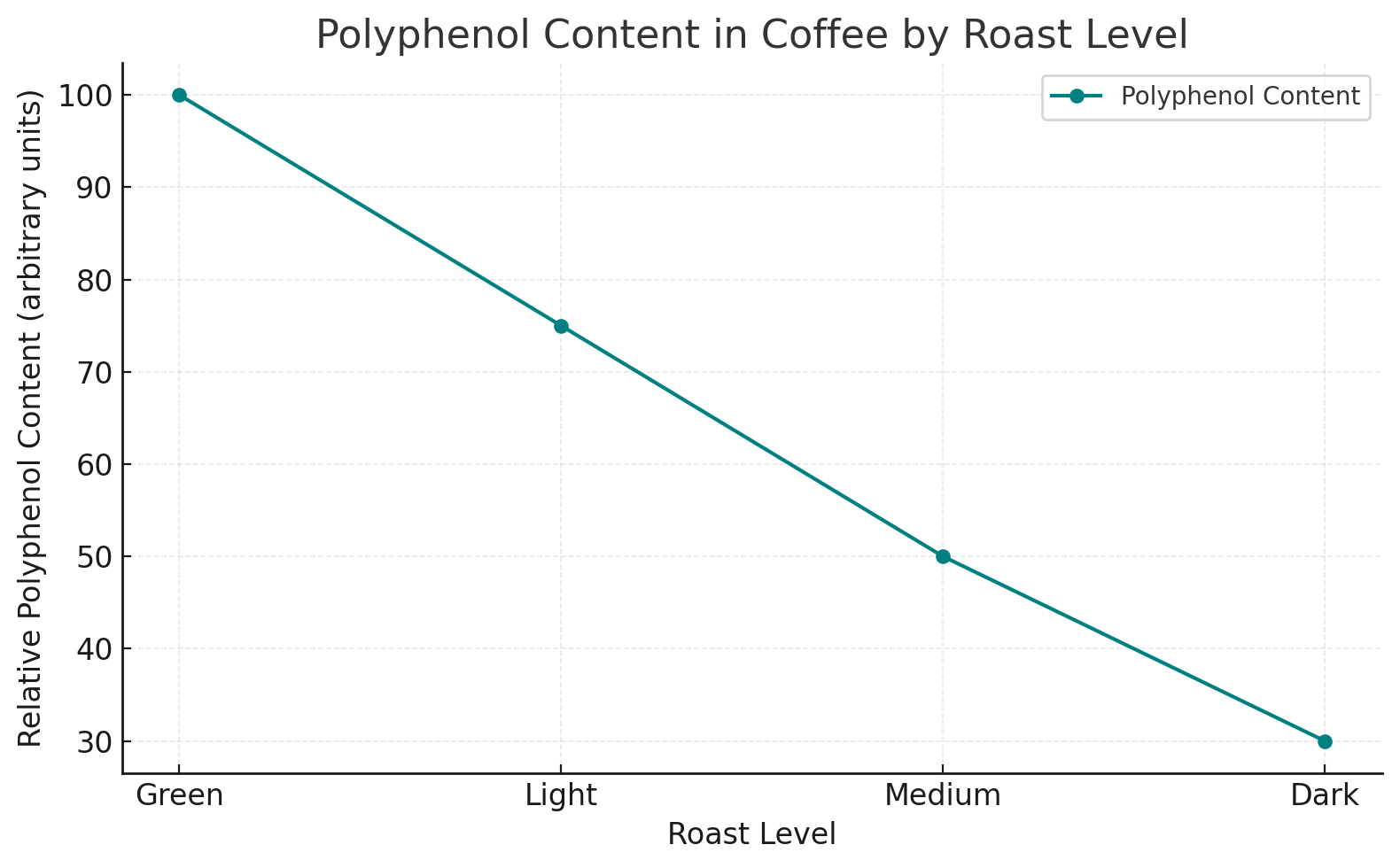
Coffee is one of the richest sources of polyphenols, especially chlorogenic acid, contributing to its bright acidity and antioxidant effects.
The level of polyphenols can vary based on the roast profile and brewing method.
This chart depicts polyphenol content across different coffee roast levels.
What is Chlorogenic Acid?
Chlorogenic acid is a natural antioxidant in coffee beans, especially green (unroasted) coffee. It is one of the primary compounds contributing to coffee's acidity and fruity notes, particularly in lighter roasts. The name comes from the Greek words chlorosis (green) and genes (to produce), hinting at its abundant presence in green coffee.
The Roasting Process: A Transformational Journey
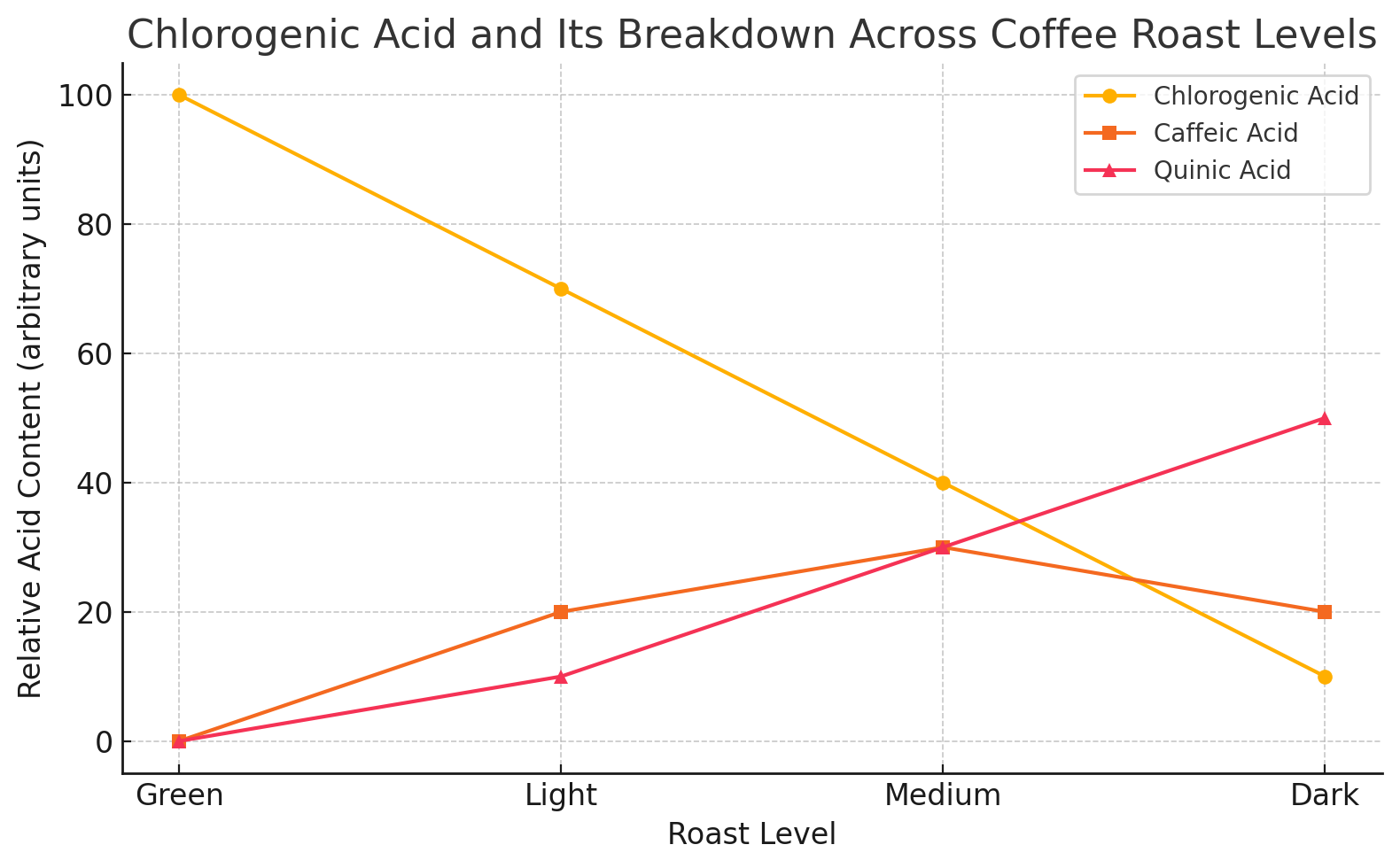
During roasting, chlorogenic acid undergoes a fascinating transformation. As the coffee beans are exposed to high temperatures, chlorogenic acid breaks down into two key components:
Caffeic Acid – known for its antioxidant properties and mildly acidic taste.
Quinic Acid – responsible for the bitter and sometimes astringent notes in darker roasts.
This breakdown explains why lighter roasts have more pronounced acidity and fruity flavors, while darker roasts exhibit a bolder, bitter profile.
Chlorogenic Acid Affects Coffee Flavor
Light Roasts: High levels of chlorogenic acid contribute to a bright, zesty acidity with floral and fruity undertones.
Medium Roasts: As the roast progresses, chlorogenic acid breaks down, balancing acidity with slight bitterness.
Dark Roasts: Much of the chlorogenic acid has decomposed, leading to the formation of quinic acid, which can create a heavier, more bitter profile.
Here's a visual showing how chlorogenic acid decreases as roast level increases, while its breakdown products—caffeic acid and quinic acid—rise and change in flavor impact.
Health Benefits of Chlorogenic Acid
Beyond flavor, chlorogenic acid is celebrated for its health benefits:
Antioxidant Powerhouse: Helps neutralize free radicals and reduce inflammation.
Metabolic Support: May aid in blood sugar regulation and fat metabolism.
Brain Health: Potential neuroprotective effects that can improve cognitive function.
Brewing Methods: Maximizing Chlorogenic Acid
To make the most of chlorogenic acid in your coffee:
Opt for light to medium roasts to retain their fruity acidity.
Use brewing methods like pour-over or Aeropress, which emphasize clarity and acidity.
Avoid over-extraction, as this can produce more quinic acid, increasing bitterness.
Start the Conversation
Next time you sip your morning coffee, take a moment to savor those bright, zesty notes. That’s chlorogenic acid at work — a tiny compound significantly impacting flavor and health.
Which roast profile do you prefer — bright, acidic, bold, or bitter?
References:
Clifford, M. N. (2000). "Chlorogenic acids and other cinnamates – nature, occurrence, dietary burden, absorption and metabolism." Journal of the Science of Food and Agriculture, 80(7), 1033-1043.
Farah, A., & de Paula Lima, J. (2019). "Chlorogenic acids in coffee: Analysis, structure, and bioavailability." Food Research International, 129, 108736.
Mussatto, S. I., et al. (2011). "Production, Composition, and Application of Coffee and Its Industrial Residues." Food and Bioprocess Technology, 4(5), 661-672.
Petracco, M. (2005). "Brewing: The Chemistry of Coffee Extraction." In Coffee: Recent Developments (pp. 250-290). Wiley-Blackwell.
International Coffee Organization (ICO). (2023). "Chemical Compounds in Coffee." Website